현재 위치:홈 > 뉴스현황 > Press Events > New Technique Enable...
저자: 업로드:2017-07-04 조회수:
If you’re reworking a genome, you might want to heed the old saying, “Measure twice, cut once.” Otherwise, your attempts to right the genome or modify it for special purposes could end in genomic wrongs—off-target effects. For example, the popular gene-editing tool known as CRISPR could go astray, altering genes other than the ones it was meant to alter. If only CRISPR’s potential slips could be foreseen! Then, perhaps, they could be avoided, and CRISPR would realize its potential not only in research, but also in medicine.
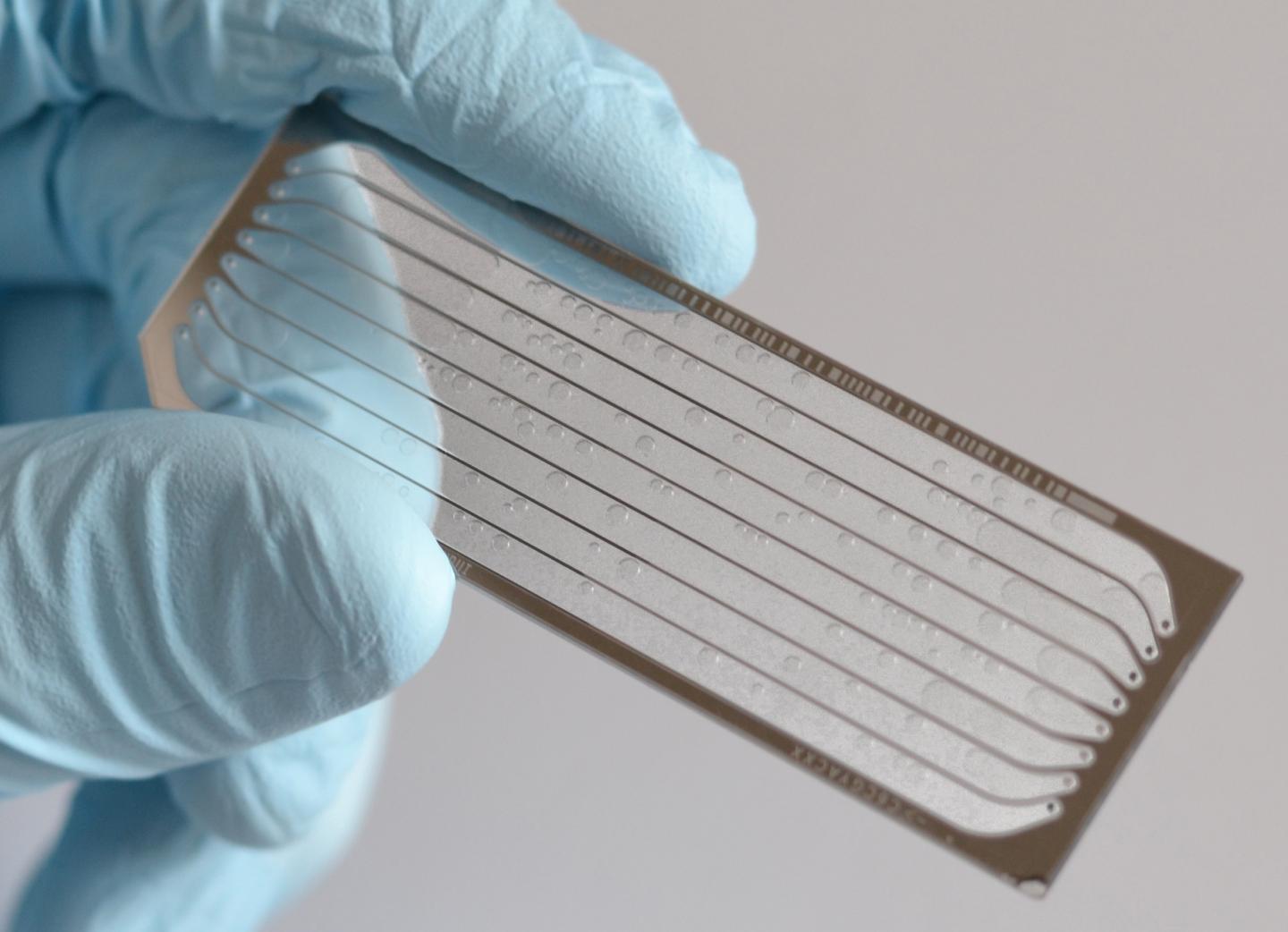
Scientists from The University of Texas at Austin took an important step toward safer gene-editing cures for life-threatening disorders, from cancer to HIV to Huntington's disease, by developing a technique that can spot editing mistakes a popular tool known as CRISPR makes to an individual’s genome. It repurposes next-generation sequencing chips to enable the massively parallel profiling of protein–nucleic acid interactions.
The scientists used CHAMP to provide the first comprehensive survey of DNA recognition by a type I-E CRISPR-Cas (Cascade) complex and Cas3 nuclease. CHAMP, the scientists showed, was able to simultaneously measure the interactions between proteins and ∼107unique DNA sequences.
Additional details appeared June 29 in the journal Cell, in an article entitled, “Massively Parallel Biophysical Analysis of CRISPR-Cas Complexes on Next Generation Sequencing Chips.” These details suggest that CHAMP provides a framework for high-throughput, quantitative analysis of protein–DNA interactions on synthetic and genomic DNA.
“Analysis of mutated target sequences and human genomic DNA reveal that Cascade recognizes an extended protospacer adjacent motif (PAM),” the article’s authors wrote. “Cascade recognizes DNA with a surprising 3-nt [nucleotide] periodicity. The identity of the PAM and the PAM-proximal nucleotides control Cas3 recruitment by releasing the Cse1 subunit.”
Essentially, these findings led to a model for the biophysical constraints governing off-target DNA binding. This model, for example, suggests that Cascade pays less attention to every third letter in a DNA sequence than to the others.
"So, if it were looking for the word 'shirt' and instead found the word 'short,' it might be fine with that," explained Stephen Jones, Ph.D., a postdoctoral researcher at UT Austin and one of three co-lead authors of the Cell paper.
Knowing such rules could lead to better computer models for predicting which DNA segments a specific CRISPR molecule is likely to interact with. And that can save time and money in developing personalized gene therapies.
"You and I differ in about 1 mill