
서비스분야
현재 위치:홈 > Services & Solutions > Integrated Projects > Antibody Drug Antibo...
Each ADC R&D project is its own challenge due to the varieties in the assembly of ADC molecules. With this concept in mind, Medicilon promises careful planning, meticulous execution and accurate results through years of practical experience and effective communication with our clients.
Medicilon offers clients with a pool of payload drugs of various mechanisms. We also offer custom synthesis of payload drugs as clients demands.
One important pharmacological parameter of an ADC is the in vivo efficacy that directly reflects its potency and influences clinical trial designs. Our animal models are all established and maintained under the regulation of AAALAC. We conduct our tests with GLP-like standards.
We have established more than 200 types of oncological models for ADC efficacy assessment.
ADC raises the difficulties of PK study for each component ADC molecules owning unique PK characteristics. Medcilon provides high quality quantification assays for key parameters in ADC PK study, presenting accurate results.
| Analyte | Description | Common analysis methods |
| Conjugated Antibody | Antibody with minimum of DAR>=1 | LBA |
| Total Antibody | Conjugated, partially unconjugated and fully unconjugated(DAR>=0) | LBA |
| Small Molecules | Released/free samllmolecule and its metabolities | LC-MS/MS |
| ADA | Antibodies against antibody of ADC, linker or drug | LBA |

Developing stable and reliable methods for results with high correlation.
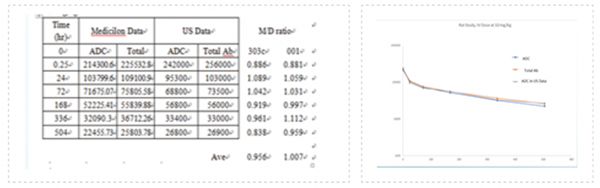
Benchmarking with global lab standard for results with high consistency.
Immunogenicity is a key parameter when evaluating biologic therapeutics. It could increase the risk for adverse effect and reducing ADC efficacy. Medicilon fully understands the compexity of ADA evaluation and offers our clients with comprehensive immunogenicity assays.
Medicilon offers rigorous and specific safety assessment services strictly following S6 & S9 Regulation of ICH and in compliance with the requirement of NMPA, FDA, OECD and TGA.
 관련 실험실
관련 실험실





