현재 위치:홈 > 뉴스현황 > Press Events > 3 Bispecific Antibod...
저자:medicilon 업로드:2021-11-26 조회수:
On October 19, Bio-Thera Solutions Ltd., (Bio-Thera) a new bispecific antibody drug BAT7104, obtained the license for clinical trials.
On October 21, Kintor Pharmaceuticals’ (Kintor) PD-L1/TGF-β dual target antibody (GT90008) was approved for clinical use.
On October 28, HCW Biologics Inc.’s (HCW) fusion protein complex HCW9218 was approved by the FDA for cancer treatment trials.
In just 10 days, Medicilon helped 3 bispecific antibody therapeutic drugs to be approved! This is another achievement of the high-quality construction of Medicilon's one-stop preclinical medicine R&D service platform. Behind these remarkable achievements, it is inseparable from the efforts and dedication of every researcher in Medicilon.
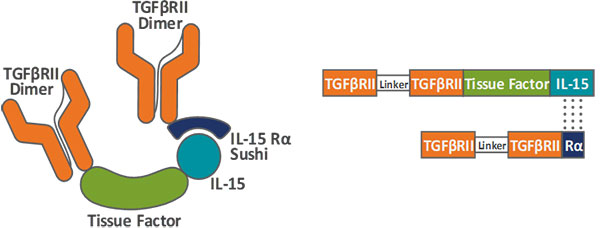
“HCW9218 is a special preparation with many targets and interference factors, making it much more difficult to develop than general innovative drugs. This is a challenge for researchers, and it is also an experience to surpass oneself,” said by Dr. Dengji Zhang, Director of Medicilon Biotechnology's Drug Analysis Department.
As a heterodimer, bifunctional fusion protein complex, HCW9218 contains the extracellular domain of TGF-β receptor II and IL-15/IL-15 receptor α complex, which can effectively activate and proliferate NK cells And CD8+ T cells, enhance the cytotoxicity of cells to tumor targets, optimize the efficacy of chemotherapy and reduce the side effects of chemotherapy.
As a partner of HCW, Medicilon has set up a team of experts for research and development in accordance with the principle of "case by case" in response to the HCW9218 project's many key technical points and the difficulty of research. After several changes and modifications, Medicilon finally established an analytical method suitable for HCW9218 under the GLP laboratory environment and operating specifications, provided pre-clinical pharmacokinetics and safety evaluation studies, and made every effort to promote the high-quality and efficient completion of the project.
In addition, the preclinical pharmacology and toxicology research team of Medicilon relies on the comprehensive and mature SEND data conversion platform in software, technology, specifications, quality and other aspects to help HCW9218 successfully apply for FDA and push forward to enter the clinical trial stage.
If the R&D of HCW9218 is a technical "Tough Battle", then the R&D of GT90008 is a "Competition" against time.
GT90008 is a PD-L1/TGF-β dual-target antibody, which can simultaneously inhibit the high activity of PD-L1 and TGF-βR2, and has the potential to become a best-in-class drug. The product is currently being developed for the treatment of multiple types of solid tumors. In the development of GT90008, Medicilon relied on the protein/antibody pharmacokinetics research platform and the biotechnology drug non-human primate safety evaluation technical service platform with innovative research and development technology and provide GLP-compliant (Including pharmacokinetics and safety evaluation) and comprehensive preclinical research services of pharmacokinetics. The R&D of the entire project is progressing smoothly and efficiently.
3 bispecific antibody therapeutic drugs assisted by Medicilon were approved within 10 days. In addition to Medicilon's strong R&D team, this is also due to the excellent academic atmosphere of Medicilon's R&D laboratory and the team's 17 years of accumulation. In the future, Medicilon will help more new drugs to be approved and marketed.
HCW Biologics is a transformative immunotherapy company focused on inflammation, an unresolved state of inflammation and chronic inflammation. The company is developing new immunotherapies aimed at improving the health span by breaking the link between chronic low-grade inflammation and age-related diseases such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases and autoimmune diseases. Use its TOBITM discovery platform to generate novel multifunctional fusion protein molecules with immunotherapeutic properties for the treatment of cancer, inflammation and other diseases. The two lead molecules of HCW, HCW9218 and HCW9302, are through the TOBI™ exploration platform. The FDA has approved the first human phase 1b clinical trial of HCW9218 for patients with advanced pancreatic cancer. HCW9302 is currently undergoing IND research on autoimmune indications.
Founded in 2009, Kintor Pharmaceuticals focuses on the development and industrialization of potential "best-in-class" and "first-in-class" innovative drugs, and is committed to becoming a leader in the research, development and commercialization of innovative therapies. The pioneering pharmaceutical industry has a forward-looking layout of a diversified product pipeline including small molecule innovative drugs, biological innovative drugs and combination therapies, including 7 new drug projects under clinical research, such as two androgen receptor (AR) antagonists and ALK -1 monoclonal antibody, mTOR kinase targeting inhibitor, Hedgehog/SMO inhibitor, AR-PROTAC and PD-L1/TGF-β dual target antibody, as well as the ALK-1/VEGF double antibody and c-Myc inhibitor that are undergoing preclinical research. The company has more than 80 patents that have been obtained and applied for worldwide, and many projects have been listed as special projects of the country's 12th and 13th five-year "Major New Drug Creation". On May 22, 2020, Kintor Pharmaceuticals was officially listed on the main board of The Stock Exchange of Hong Kong Limited, stock code: 9939.HK.
Since the founding of our company in 2004, Medicilon (Stock Code: 688202.SH) has grown into one of the professional drug discovery contract research organizations (CRO) in China. Over the years, Medicilon keeps improving their services in biotechnology and pharmaceutical research. Their services now span across medicinal chemistry, process chemistry, in vitro and in vivo DMPK, preclinical development and bioanalytical support. Medicilon grows together with the clients and delivers the new drug research and development services to more than 900 clients globally. Medicilon is proud to contribute to human health in the globe.