
서비스분야
현재 위치:홈 > Services & Solutions > Integrated Projects > Non-clinical Researc...
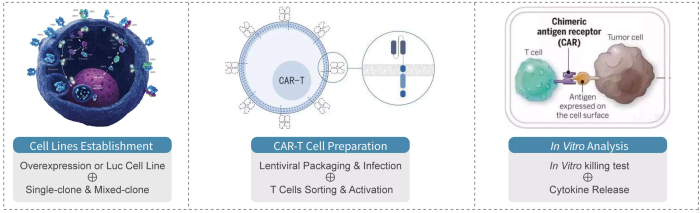
Medicilon has built a one-stop research platform for the preclinical R&D of cellular immunotherapies, covering a variety of immunotherapy methods including CAR-T, TCR-T and CAR-NK. Using a wealth of animal models and a variety of advanced analysis techniques, comprehensively considering the characteristics of different research projects, Medicilon has completed numerous pre-clinical projects for clients worldwide.
Cellular immunotherapy has developed by leaps and bounds in recent years, providing a cure for many difficult-to-treat cancers. With the continuous discovery of tumor targets and mechanisms, the track is still full of vitality and hope bringing new breakthroughs in tumor treatment and human welfare.
Medicilon has built a one-stop research platform for the preclinical R&D of cellular immunotherapies, covering a variety of immunotherapy methods including CAR-T, TCR-T and CAR-NK. Using a wealth of animal models and a variety of advanced analysis techniques, comprehensively considering the characteristics of different research projects, Medicilon has completed numerous pre-clinical projects for clients worldwide.
| 类别 | CAR-T Cell | TCR-T Cell | CAR-NK Cell |
| DNT Cell | TIL Cell |
❖ Potential undesired effects of the study drug on physiological function at doses within or above the therapeutic range
❖ Generally include effects on the central nervous system, cardiovascular system, and respiratory system
❖ Research on other organ systems may need to be supplemented depending on product characteristics
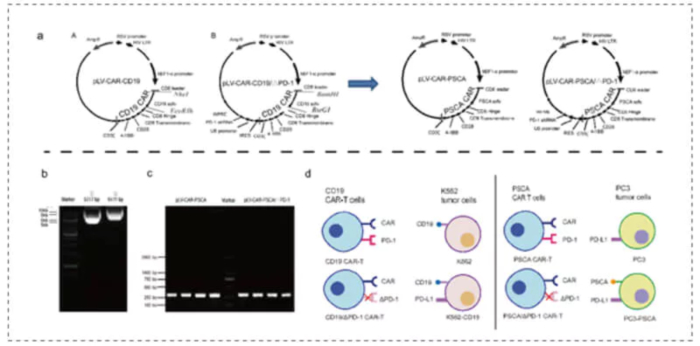
The PD-1 shRNA was integrated into the CAR plasmid, and then transduced into T cells by a lentiviral vector to obtain CAR-T cells with PD-1 silencing function. The results showed that effective silencing of PD-1 significantly suppressed the immunosuppressive effect of the tumor microenvironment and prolonged the activation time of CAR-T cells, resulting in a longer tumor killing effect. PD-1-silenced CAR-T cells significantly prolong the survival of mice with subcutaneous prostate and leukemia xenografts. This demonstrated that PD-1 silencing technology is a suitable solution to promote the therapeutic effect of CAR-T cells on subcutaneous prostate and leukemia xenografts[1]. The plasmid sequencing work in this experiment was completed by Medicilon.
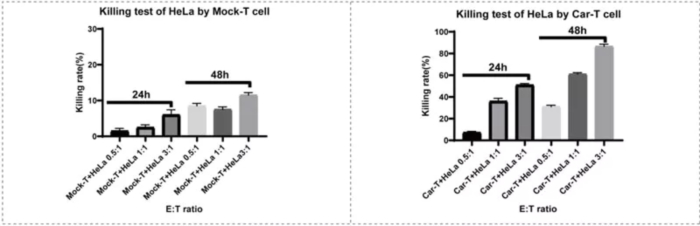
CAR-T cell killing experiments show that CAR-T cell-dependent killing is increased compared with Mock-T cells
❖ Can be prepared from blood donated by healthy volunteers
❖ Some proof-of-concept studies could be done with alternative products of animal origin
❖ The similarities and differences between non-clinical test substances and clinical samples should be explained in the new drug application
❖ Consider factors such as production process, key quality characteristics (such as titer), preparations for clinical use
❖ If there is species specificity, the activity of the test substance in non-clinical research should be evaluated
❖ If the vector uses an expression tag, the impact of the tag on the supportability of non-clinical trials should be analyzed
❖ Bioluminescent Imaging (BLI)
❖ Flow Cytometry: Detecting the number of tumor cells in animals
❖ Flow Cytometry, ELISA, MSD: Changes in tumor-related cytokines
❖ Related Parameters: Tumor volume, tumor weight, tumor cell colonization site in animals and median survival period of animals
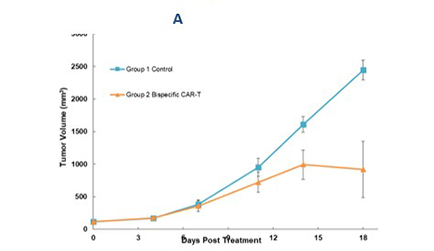
A target

B target

A target & B target
The picture shows the pharmacodynamic study of dual-target CAR-T cells in K 562 subcutaneously transplanted tumor mouse model
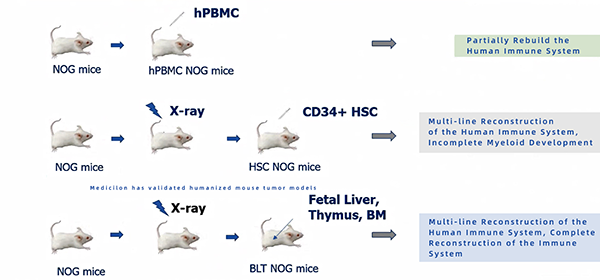

The picture shows the pharmacodynamic study of the mouse pharmacodynamic model of hPBMC immune system reconstruction induced by Raji-luc fluorescein-labeled lymphoma cells
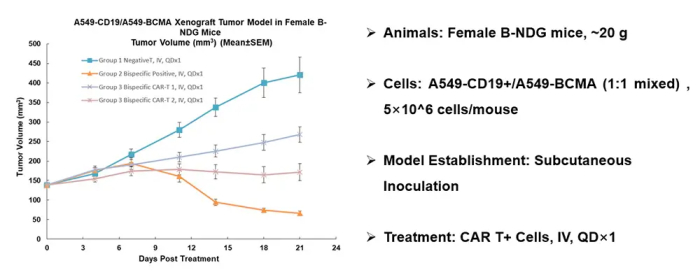
CAR-T cell killing experiments show that CAR-T cell-dependent killing is increased compared with Mock-T cells
❖ Exposure: Gene therapy products should be analyzed and evaluated according to the specific characteristics of the product considering the actual exposure in non-clinical research
❖ Biodistribution: Biodistribution of gene therapy products refers to the distribution, persistence and clearance of gene therapy products in target and non-target tissues in vivo
❖ Shedding: Shedding assays should include testing for infectivity of excreted components
❖ Imaging Technology
❖ Flow Cytometry
❖ Immunohistochemical Technique
❖ Quantitative PCR Technology
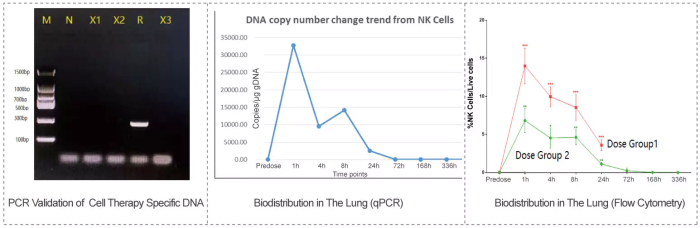
The detection results obtained by qPCR and flow cytometry are consistent



In toxicology research, a comprehensive safety analysis and evaluation of gene therapy products should be conducted, and the safety of the expression products of introduced genes should also be evaluated if necessary.Gene therapy products should be effectively introduced/exposed in relevant animal species.
The non-clinical safety studies of cellular immunotherapiesare mainly designed and carried out based on the potential safety risks of the drugs. In addition to the common safety risks, cell therapies may also contain the risks of:
❖ Cytokine Release Syndrome (CRS)
❖ Graft Versus Host Disease (GVHD))
❖ Reversible Neurotoxicity
❖ B Cell Reduction
❖ On-Target / Off-Tumor)
❖ Tumorigenic / Tumorigenic
❖ Homologous Mouse Model
❖ Transgenic Mouse Model
❖ Transplanted Tumor Mouse Model
❖ Non-Tumor-Bearing Immunodeficiency Mice
❖ HIS Mouse Model
❖ Various methods of administration such as intravenous injection, intratumoral injection, and joint cavity injection are available.
❖ Single Dose Toxicity Test
❖ Repeated Dose Toxicity Test
❖ Immunogenicity / Immunotoxicity
❖ In vitro Soft Agar Clone Growth Test
❖ In vivo Tumorigenicity / Tumorigenicity
❖ Formulation Safety
❖ Other: Express Product Safety
 관련 실험실
관련 실험실





