The solid screening platform of Medicilon process department strictly complies with the registration regulations and regulatory requirements of China NMPA, FDA and other regulatory authorities, studies and controls the solid morphology of drug molecules in API and preparations, and can formulate the optimal screening strategy according to the material characteristics, including but not limited to crystallization method, solvent selection and ion selection.
The Importance of Solid Form Screening
The solid form screening of APIs is an important tool in the early process development of new drugs. The purpose is to find as many crystal forms as possible and determine the most suitable solid form for development, so that you can:
Improve the Quality of Drugs
There are significant physical and chemical differences between different polymorphs of the same drug (hygroscopicity, physical/chemical/mechanical stability, purity and impurities). This could lead to differences in solubility, dissolution rate and bioavailability, which directly affect the quality of the final product.
Optimize Intellectual Property
As a material structure patent, crystal form is an effective tool for prolonging the life cycle of new drugs. For generic drugs, it can break through the patent barriers of the original research and obtain the right to enter the market.
Compliance with Registration Regulations
The drug regulatory laws and regulations of China, US and other developed countries have set clear requirements for the content of crystal form research in new drug clinical trial applications, new drug marketing applications, and generic drug applications, and have formulated relevant guiding principles for crystal form research.
The solid form screening platform of Medicilon Process Department strictly complies with the registration regulations and regulatory requirements of China’s NMPA, the US FDA and other regulatory agencies. It conducts research and control on the solid form of drug molecules in APIs and preparations, and can formulate the best effect based on the characteristics of the substance screening strategies, including but not limited to crystallization methods, solvent selection, and ion selection.
Service Scope of Solid Form Screening Platform
Discovery
Various Salt Forms/Crystal Forms/Eutectic of API
Evaluation
The Druggability of Different Crystal Forms of API
Development
The API crystallization production process to obtain the target crystal form, morphology and particle size
Salt Screening
Purpose
Improve Drug Properties
Discover the Most Suitable Salt Type for Development
Ensure Effective Patent Protection
Avoid Prior Technology Patents
Technology
Free State Property Characterization
Solubility in Different Solvents
Choose the Right Counter Ion
Various Salt-Forming Technologies
Characterization of Physical and Chemical Properties
Solid and Solution Stability Research
Salt Type Selection and Recommendation
Patent Disclosure Materials
Salt Type Screening Process
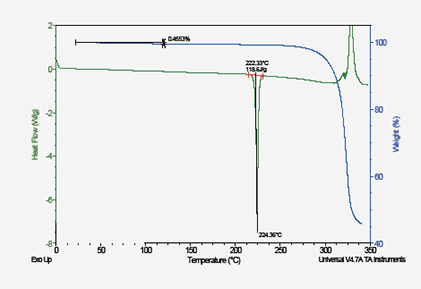
Thermal Analysis (TGA/DSC): Solvent (water) and Non-Solvate Analysis
No Crystal Type
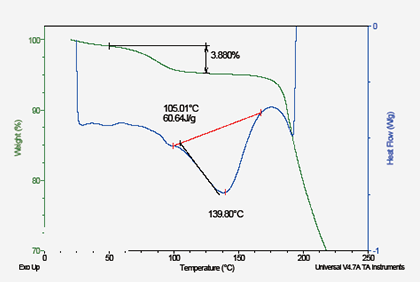
Solvent (water) Compound
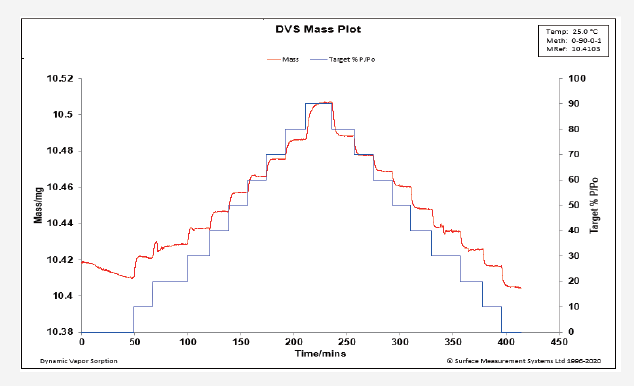
Dynamic Moisture Sorption (DVS) Applications – Inspection of Sample Moisture Absorption
Eutectic Screening
Purpose
Improve the Defects of the Free State Property
Discover the Most Suitable Eutectic Form for Development
Ensure Effective Patent Protection
Avoid Prior Technology Patents
Polymorph Screening
Purpose
Discover the Most Suitable Crystal Form for Development
Ensure Effective Patent Protection
Avoid Prior Technology Patents
Technology
Analysis of Existing Technologies
Multiple Crystallization Techniques
Characterization of Physical and Chemical Properties
Solid and Solution Stability
Analysis of the Relative Stability of Polymorphs
Crystal Type Selection and Recommendation
Patent Disclosure Materials
Polymorph Screening Process
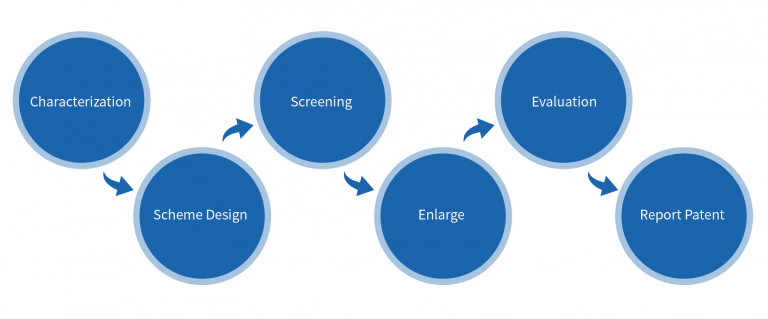
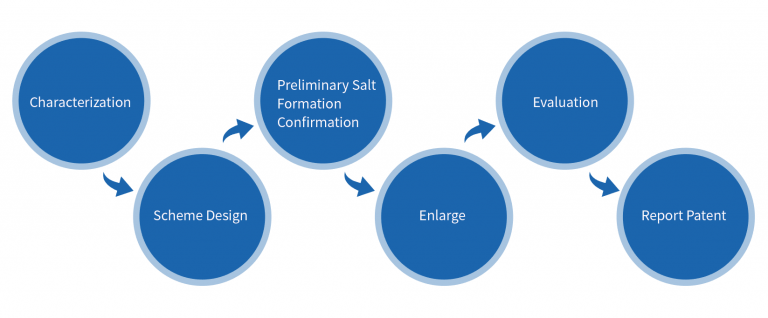
Characterization→Scheme Design→Screening→Enlarge→Evaluation→Report Patent
Development and Verification of Qualitative, Quantitative and Limit Analysis Methods
Raw Material Crystallinity or Impurity Crystal Type Analysis
Qualitative and Quantitative Analysis of Crystal Forms of Raw Materials in Preparations
Development and Verification of Particle Size Distribution Analysis Method
X-Ray Powder Diffraction Method (XRPD)
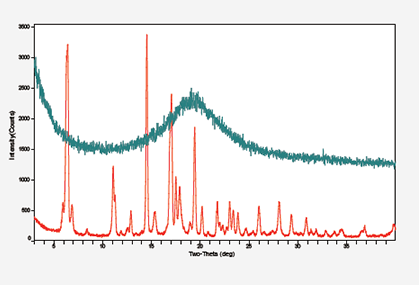
Crystallinity Check
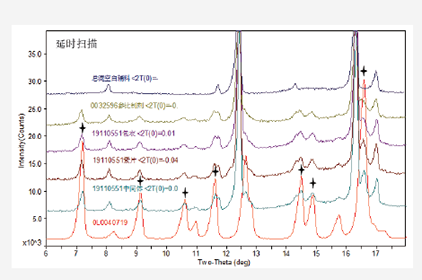
Qualitative Analysis of Crystal Forms of Raw Materials in Preparations
 관련 실험실
관련 실험실








 관련 실험실
관련 실험실





