
서비스분야
현재 위치:홈 > Services & Solutions > Integrated Projects > Nucleic Acid Drug R&...
Medicilon nucleic acid drug R&D platform is an integrated and comprehensive solution that covers drug discovery, CMC and preclinical research services. Based on a rigorous scientific attitude, an open-minded scientific spirit and advanced equipment, our integrated solution can meet the industry's research and development needs for cutting-edge innovative nucleic acid drugs, and undertake research programs like nucleic acid drug discovery, screening and preclinical research services for pharmaceutical companies and scientific research institutions.
Medicilon nucleic acid drug R&D platform is an integrated and comprehensive solution that covers drug discovery, CMC and preclinical research services. Based on a rigorous scientific attitude, an open-minded scientific spirit and advanced equipment, our integrated solution can meet the industry's research and development needs for cutting-edge innovative nucleic acid drugs, and undertake research programs like nucleic acid drug discovery, screening and preclinical research services for pharmaceutical companies and scientific research institutions.
For its fast and intuitive design of base sequences, the development of nucleic acid drugs applies simple materials, convenient preparation processes and affordable production costs, which greatly shorten the drug development cycle, making it possible to customize individualized treatment plans. Hence, it offers a feasible solution for rare diseases and other problems currently plagued.
◆ Monomer synthesis
◆ Oligonucleotide synthesis
◆ Delivery system synthesis
◆ Oligo conjugate synthesis
◆ Sugar modification
◆ Nucleobase modification
◆ Backbone modification
◆ Delivery system modification

◆ Evaluation of binding between siRNA-GALNAc and targeted liver cells. (ELISA,SPR,FP,FACS, MSD, Confocal microscope)
◆ Evaluation of decrease in target mRNA/protein level.
◆ Evaluation of cell phenotypes and functional interfere.
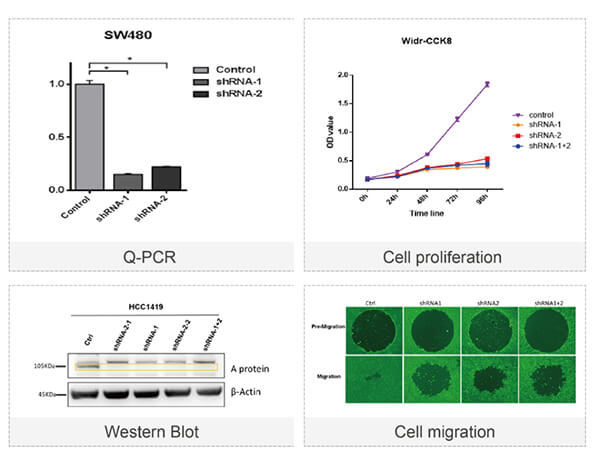
◆ Evaluation of cell phenotypes and functional interfere.
◆ Searching for potential off-target mRNA/protein in the database, such as NCBI, nucleotideBLAST.
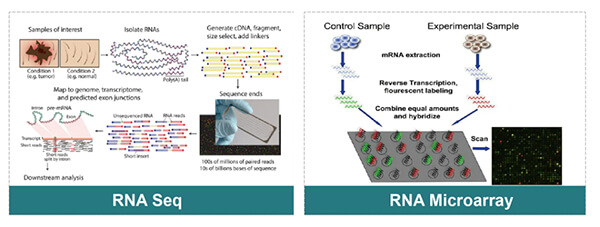
◆ Overall analysis applying RNAseq or RNA Microarray.
• Select starting materials:We aim to choose starting materials with traits like easy to purchase, mild toxicity, good quality stability;
• Process R&D of nucleic acid:We aim to develop stable and green synthesis routes with low cost and high security.
• Quality Control:Up-to-date quality control system with complete technical standard.
• Scale up:End to end service ensuring smooth transfer.
Due to their low immunogenicity, biocompatibility, and high encapsulation efficiency for oligonucleotide molecules, lipids and their derivatives have become the go-to delivery systems for nucleic acid drugs that have attracted much attention in recent years. The system is positively charged in the physiological environment. The negatively charged nucleic acid molecules are encapsulated by electrostatic action, and the positively charged surface can also help the entire carrier system to combine with the cell membrane of the target cell, thereby playing a delivery role.
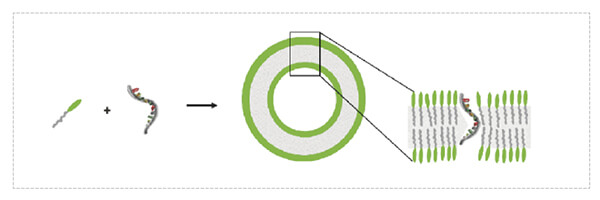

• Easily modified, easily synthesized, easily produced.
• The on-target and off-target ratio of delivery should be within an acceptable range.
• The effective dose must be significantly lower than the toxic dose.
• The bioactivity of the nucleic acid should be consistent from batch to batch.
• In most clinical cases, repeated administration does not result in loss of efficacy or safety.

| Nucleic acid-lipid system R&D |
• Formulation: drug to lipid ratio, solvent screening, aqueous to organic solvent ratio • Process: Preparation methods • Stability • Dosage form screening |

| PK/TK analysis |
• H-ELISA LC-MS/MS; • H-ECL; • RT-qPCR; • qPCR; • ddPCR |
| ADA analysis |
• Total ADA; • MSD ; • Nab analysis: CLBA or cell-based assay |
| PD or TOX related cytokines & biomarkers |
Cytokine&Biomarker • Singleplex; • Multiplex (Luminex, MSD, FACS CBA) • FACS |

• High specificity
• High sensitivity: ng level
• Advantages: end product detectable

• High specificity
• Sensitivity: Detectable within 1 log copy
• Advantage:More Sensitive

• Sensitivity: pM level
• Advantages: variable marking strategy; personalized reaction strategy.
◆ Contrast of different drug delivery methods.
◆ Relevance analysis between pharmacology and target mRNA/protein degradation & nucleic acid drug PK
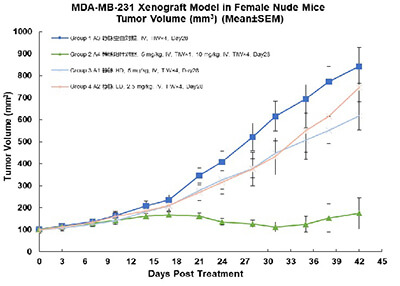
• Animals: Female BALB/c Nude mice;
• Cells: MDA-MB-231, 5*106/mouse;
• Model Establishment: Right flank SC injection;
• Treatment: IV injection;
TIW (three times a week);
Group3, 4: mRNA (LNP)group.

• Animals: Female BALB/c Nude mice;
• Cells: MDA-MB-231, 5*106/mouse;
• Model Establishment: Right flank SC injection;
• Treatment: Intratumor in-jection;
TIW (three times aweek);
Group 7, 8: mRNA(LNP) group.
◆ Dendrimer LNP
◆ Luc mRNA;Ⅳ and intra-tumoral

◆ CNP-generated exosome
◆ Delivery of PTEN mRNA by Glioma-directed Exosome EXO-T,IV

| Cancer Type | Cell Lines |
|---|---|
| Bladder Cancer | MB49 |
| Brain Cancer | G261 |
| Breast Cancer | 4T1, EMT6, JC,EO771 |
| Colon Cancer | CT26.WT, MC-38, Colon26 |
| Leukemia | C1498, L1210, WEHI-3 |
| Liver cancer | H22, Hepa 1-6 |
| Lung Cancer | LLC1, KLN205 |
| Lymphoma | A20, EL4, L5178-R, E.G7-OVA |
| Mastocytoma | P815 |
| Melanoma | B16-F10, Clone-M3 |
| Myeloma | J558 |
| Pancreas Cancer | Panc 02 |
| Renal Cancer | RENCA |
| Luciferase Cell Line |
| G261-luc, 4T1-luc, Mc38-luc, H22-luc, B16-F10-luc, LLC1-luc |
| Cancer Type | Cell Lines in PBMC or HSC CD34+ Humanized Mice |
|---|---|
| Brain Cancer | U-87 MG |
| Breast Cancer | HCC1954, MDA-MB-231, JIMT-1 |
| Colon Cancer | HT29, LoVo, Ls174T, HT-15 |
| Gastric Cancer | NCI-N87, NUGC-4 |
| Leukemia | THP-1 |
| Lung Cancer | HCC827, NCI-H1975, NCI-H292, A549 |
| Lymphoma | Raji, TMD8, MOLM-13 |
| Melanoma | A375 |
| Myeloma | RPMI-8226, NCI-H929, MM.1S |
| Ovarian Cancer | OVCAR-3 |
| Pancreatic Cancer | Capan-2 |
| Renal Cancer | 786-O |
| Skin Cancer | A431 |
 관련 실험실
관련 실험실





